Official NASA research
 NASA conducted several studies using hydrogen as a supplemental fuel on a gasoline-powered internal combustion engine. Their research demonstrated that the higher flame velocity of hydrogen was responsible for significant fuel savings and more efficient engine operation. Combustion with hydrogen has the potential to produce lower emissions and better thermal efficiency for several reasons:
NASA conducted several studies using hydrogen as a supplemental fuel on a gasoline-powered internal combustion engine. Their research demonstrated that the higher flame velocity of hydrogen was responsible for significant fuel savings and more efficient engine operation. Combustion with hydrogen has the potential to produce lower emissions and better thermal efficiency for several reasons:
1. excess oxygen in the combustion chamber further oxidizes unburned Hydrocarbons (HC) and carbon monoxide (CO);
2. excess oxygen lowers combustion temperatures which inhibits the formation of nitrogen oxides;
3. lower combustion temperatures increase the specific heat ratio of the mixture by decreasing net dissociation losses;
4. As the specific heat ratio increases, the thermal efficiency of the cycle also increases, which makes it possible to achievebetter fuel economy.

CALTECH - California Institute of Technologyalso researched to calculate the thermal efficiency and fuel economy of the engine using supplemental hydrogen. The overall efficiency of the engine increases and is greater than the energy loss incurred during hydrogen production, which improves fuel economy for the engines.

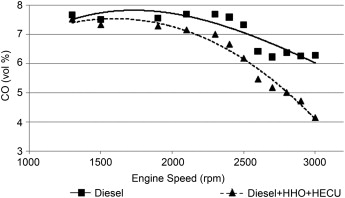
Motor torque
On average, a 19.1% increase in engine torque is achieved using hydrogen HHO compared to running on pure diesel. The increase in power is due to the concentration of oxygen in the HHO gas and a better mixing of the HHO with the air and fuel, which gives better performance.
 Engine temperature
Engine temperature
The high flame speed of hydrogen allows a reduction in ignition delay and combustion time, which reduces heat loss and allows a higher compression ratio with better thermal efficiency.The result is a lower engine temperature.

Engine noise and vibration
The higher combustion rate of hydrogen makes it possible to increase more quickly the pressure and temperature of the engine inminimizing vibration and noise, especially at idle.
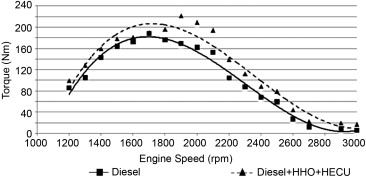
 Fuel consumption
Fuel consumption
An average gain of more than 20% is obtained using the HHO system. The reduction in fuel consumption is due to the uniform mixing of HHO gas with air (high diffusivity of HHO) because the greater presence of oxygen allows better combustion. The greatest savings occur at high speeds, as it is difficult to fully burn diesel in lean burn conditions.Hydrogen with this higher flammability will help our fuel to be burned more quickly and completely, with an associated energy gain.
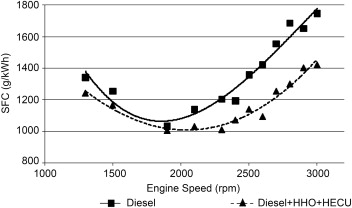

Polluting emissions
An average reduction of 35% is achieved for carbon monoxide (CO) emissions at medium and high engine speeds. The absence of carbon and the presence of oxygen in HHO gas are the main reasons for the reduction of CO. The high flammability of HHO gas allows the engine to be operated at lower loads. The HHO+fuel mixture burns faster and more completely than fuel alone, witha reduction in the associated polluting emissions.